Bringing volume to standard online conditions. A sample of the problem of calculating the volume of air required for chemical analysis and bringing it to normal conditions
ABOUT ENSURING UNITY OF MEASUREMENTS AND CONTROL IN ECOLOGY
Nikolaev Yuriy Nikolaevich 1, Kashcheev Stanislav Nikolaevich 2, Gomyranov Peter Vladimirovich 3
1 FSUE NIIEA, Ph.D. in Physics and Mathematics. Sciences, Head. Department of Ecology FSUE NIIAE, Academician of the International Academy of Authors of Scientific Discoveries and Inventions of the Russian Academy of Natural Sciences, Academician of the Russian Academy of Natural Sciences, Corresponding Member. Academy of Engineering Sciences. A.M. Prokhorov
2 FSUE "Acoustical Institute. academician Andreev NN ", candidate of technical sciences, senior researcher of the FGUP" Academician Andreev NN »
3 FSUE NIIAE, Senior Researcher of the Laboratory of Tests of the Department of Ecology of FSUE NIIEA
Therefore, this is one of the most common ways of producing ozone. Other would be industrial furnaces, automobile engines among others that produce gas. In the lower atmosphere, ozone reacts and promotes industrial pollution of the atmosphere, counting the poison.
After release into the atmosphere, the propellant begins to spread through the free atmosphere and is transferred by convective lifts, until the high atmosphere is scattered all over the planet. In a high atmosphere, there are high-speed air currents, very powerful jet streams, the direction of which is horizontal. They spread gases in the region in all directions.
annotation
The article discusses some topical aspects of metrological support for legal norms and rules governing air quality measurement procedures and their interrelations with environmental and hygienic standards. The following aspects of the problem are considered: definitions of measurement and control concepts (PPM and MPC), normal (standard conditions) in measurements, differences in industry standards for normal conditions, reduction of the measured concentrations of polluting chemicals in the air to normal conditions, international standards and regulations in the field of protection environment to ensure the uniformity of measurements.
ON ASSURANCE OF MEASUREMENT AND CONTROL ENVIRONMENT
Nikolaev Yuri Nikolaevich 1, Kashcheev Stanislav Nikolaevich 2, Gomyranov Petr Vladimirovich 3
1 FSUE NIIAE, candidate of physics and Mathematics. Sciences, Head. Department of Ecology FSUE NIIAE, the International Academy of Authors of Science Discoveries and Inventions of Natural Sciences, Academy of Natural Sciences, prof. Academy of Engineering Sciences. AM Prokhorov.
2 N. N. Andreyev Acoustics Institute, Ph.D., senior researcher of FSUE "Acoustics Institute, Academician NN Andreev
3 FSUE NIIAE, Senior Researcher, Laboratory Test Environment Division FSUE NIIAE
Solar energy at the ultraviolet wavelength forms ozone molecules. This process occurs when you divide oxygen molecules into free oxygen atoms, recombining them with oxygen molecules through ultraviolet radiation. The chlorine atom is a powerful catalyst that breaks down ozone molecules, remaining intact throughout the process. When in high atmosphere chlorine takes many years to descend into the lower atmosphere. During this period, each atom of chlorine will destroy millions of molecules of ozone.
Therefore, the result of the reaction is. This means that we had three molecules of oxygen, and the chlorine atoms were regenerated to destroy two more ozone molecules at a time and so on, endlessly, until Chlorine sank into the lower atmosphere. It is such a rare and precious gas in the upper atmosphere that if the ozonosphere has been raised to sea level under normal conditions of temperature and pressure, this layer will reach a thickness of only a few millimeters. It is this gas that protects us from the fact that our skin is cauterized by ultraviolet radiation from the sun.
Abstract
The article discusses some of the important aspects of the metrological provision of legal regulations and regulations governing the procedures for measuring air quality and their relationship with environmental and health standards. Includes the following aspects: the definition and measurement of control (PPM and MPC), normal (standard conditions) in measuring the differences in standards-normal conditions, leading to the right conditions of the measured concentrations of contaminants in the air, international standards and regulations in the field of environment.
- 1. Unity of measurement and control: units of measurement PPM, mg / m 3 and MPC.
Operating systems of units of measurements of air quality parameters.
1.1. General definition of PPM.
To determine air quality parameters, the main units of measurement are the volume or mass fraction of the main air components, the volume fraction of gaseous pollutants, the molar fraction of gaseous pollutants, expressed as a percentage, millionths (ppm), billionths (ppb), respectively, and the mass concentration of gaseous pollutants , expressed in mg / m 3 or μg / m 3. According to the standards, the use of relative units (ppm and ppb) and absolute units (mg / m 3 and μg / m 3) is allowed when presenting measurement results in the field of air quality control. We give some definitions:
People with dark skin are not free from this cancer, the difference is only the time of exposure. The hole in the ozone layer has far greater consequences than skin cancer in humans. The standard for measuring ozone is made in accordance with its concentration per unit volume, which, in turn, receives the nomenclature of the Dobson Unit.
The death of these microorganisms leads to a decrease in the ability of the oceans to extract carbon dioxide from the atmosphere, contributing to global warming. With the death of phytoplankton, zooplankton does not survive. Without zooplankton, krill ceases to exist, reducing the fish population in the oceans, and so on. so the ozonosphere is the original, so that there is life on the planet Earth.
PPM, as well as percentage, per mil - the dimensionless ratio of the physical quantity to the same value taken as the initial value (for example, the mass fraction of the component, the molar fraction of the component, the volume fraction of the component).
PPM is the value determined by the ratio of the measured entity (substance) to one millionth of the total, where the measured substance enters.
PPM has no dimensionality, since it is a relative value, and is convenient for estimating small shares, since it is less than a percent (%) 10,000 times.
"PPMv (parts per million by volume) is a unit of concentration in parts per million by volume, that is, the ratio of the volume fraction to everything (including this fraction). PPMw (parts per million by weight) is a unit of concentration in parts per million by weight (sometimes referred to as "by weight"). Those. the ratio of the mass share to everything (including this share). Note that in most cases, the undefined unit "PPM" - for gas mixtures it is PPMv, and for solutions and dry mixtures it is PPMw. Be careful, because if you fail to determine, you may not even fall into the order of a true value. " This link to the ENGINEERING Handbook. .
1.2. PPM in gas analysis.
Let us return once again to the general definition of PPM as the ratio of the number of some units of measurement of a part (share) to one millionth of the total number of the same units as a whole. In gas analysis, this unit is often the number of moles of substance
where m is the mass of the chemical pollutant (ZXB) in the air when the concentration is measured, and M is the molar mass of this substance. The number of moles is a dimensionless quantity, it is an important parameter of Mendeleev's law for ideal gases. With this definition, the mole is a universal unit of the amount of a substance more convenient than a kilogram.
1.3. How units of concentration are related in ppm and mg / m 3.
We quote from the text:
"Note that units of concentration, referred to as ppm (parts per million), are fairly widespread; with respect to the concentration of any substance in the air; ppm should be understood as the number of kilomoles of this substance, which accounts for 1 million km of air. "(There was an error in the translation: it should read 1 millionth part of the kilometer). Further:
"To recalculate ppm in mg / m 3, it is necessary to take into account the molar mass of pollutant M s (kg), the molar mass of air M (at normal conditions, 29 kg) and its density
ρ of air (under normal conditions, 1.2 kg / m 3). Then
C [mg / m 3] = C * M zxv / (M air / ρ air) = C * M zxv / 24.2 »(1)
Let us explain the reduced concentration conversion formula.
Here C [mg / m 3] is the ZXB concentration at the measurement point with the meteorological parameters: temperature T and pressure P, and M air / ρ air = 24.2 is the normative parameter.
The question arises: when calculating the regulatory parameter (M air / ρ air) = 24.2 and air density ρ (1.2 kg / m 3), what were the values of the parameters T 0 and P 0 taken as "normal conditions"? Since for true normal conditions
T = 0 0 C, and 1 atm. ρ 0 air = 1.293 and M air = 28.98, (M air / ρ 0 air) = 28.98: 1.293 = 22.41 = V 0 (molar volume of an ideal gas), calculate the value of the "normal temperature" in (1) by the formula for reducing the density parameter [ 3]:
ρ air = ρ 0 air * f, = ρ 0 air * f = P 1 T 0 / P 0 T 1, (2)
where f is the standard recalculation coefficient of reduction to normal conditions. ρ air = M of air: 24.2 = 1.2,
f = ρ air: ρ 0 air = 1.2: 1.293 = 0.928, which corresponds to the measurement conditions
t = 20 0 C, P 0 = 760 mm Hg. Art. Therefore, in the report and the conversion formula (1), the normal conditions are T 0 = 20 0 C, P 0 = 760 mm Hg. Art.
1.4. Which determination of concentration in ppm is used in the EU-Russia program report.
The question that needs to be clarified is: which definition of ppm is taken as the basis for: ratio by volume, by weight or by moles? We show further that there is a third variant. This is important to understand, since it is a report
According to the international program "EU-Russia. Harmonization of environmental standards "and in the preamble to the report it is necessary to discuss the presented materials.
We rewrite formula (1) for the reverse calculation:
C = (C [mg / m 3] * M air) / (ρ air * M зхв) =
(C [mg / m 3] / M зхв) / (ρ air / М of air) = k * С [mg / m 3] * / M зхв,
where k = M air / ρ air = 29. / 1.2 = 24.2 (2 ')
In formula (2 '), the relative concentration of C is the ratio of the number of moles of impurity (DXB) and air under normal conditions. Let us explain this statement, starting from the definition of the PPMW value:
Cw = n / (n 0/10 6) = 10 6 n / n 0 (3)
n is the number of kilograms of ZHB in a certain volume under the conditions of measurement,
n 0 is the number of kilometers of air under normal conditions in the same volume.
Since n = m / M * sxv and n 0 = m 0 / M * 0, where M * sxv and M * 0
molar masses of pollutant and air, we obtain the expression for Cw:
Cw = 10 6 (m / M * s) / (m 0 / M * 0) =
10 6 ((m / V 0) / M * sxv) / ((m 0 / V 0) / M * 0) = 10 6 (C zxv / M * sxv) / (C 0 / M * 0), ( 4),
where V 0 is the molar volume of air.
Expression (4) coincides with the reduction formula (2),
since (m / V 0) = C xx = 10 6 C [mg / m 3] and (m 0 / V 0) = C 0 = ρ of air
(under normal conditions, 1.2 kg / m 3), V 0 = 22.4 [L] and M 0 = M of air = 29 [kg], which proves our assertion about the definition of Cw.
1.5 Let's consider one more definition of PPM for analysis of ZXB in air in accordance with the general definition, namely: ppm ism = Cw:
Cw ism = 10 6 n zxv / n air, where (5)
n ism - the number of kilos of ZHB in a certain volume under the conditions of measurement,
n air = is the number of kilometers of air under the conditions of measurement in the same volume.
The formula (4) for measuring pm in this case takes the form:
Cw = 10 6 (C xx / M * sxv) / (C air / M * 0) (5 ')
The air concentration at the measurement point C air = m air / V 0 is related to its density (concentration) by the expression (2): With air = C 0 *f , With air = ρ air . (2’)
Substituting (2 ') in (5'), we obtain (since (C sxB / f) = C 0 SXB):
Cw = 10 6 (C xx / M * sxv) / (C 0 * f / M * 0) = 10 6 ((C sxv / f) / M * sxv) / (C 0 / M * 0) = C 0 w,
which is the normative value of ppm, reduced to normal conditions.
Consequently, the definition of Cw introduced by the definition of 1.5 coincides with C 0 w and it does not require any correction for reduction to normal conditions, since it is identically equal to. The conclusion is quite obvious, since the ratio of the measured ZHV and air is used under the same measurement conditions.
It is important to note that in the standard concerning the verification circuit for the means of measuring components in gaseous media it is shown that a unit of the molar fraction or mass concentration of the components is transferred from working standards of different digits to all types of measuring instruments intended for assessing the quality of atmospheric air and air in the work area.
3. Normal (standard) conditions for measuring air parameters.
3.1. When measuring atmospheric air, air in the working area and also industrial emissions and hydrocarbons in gas mains, there is a problem of bringing the volume of measured air to normal (standard) conditions. Often in practice, air quality measurements do not use recalculation of measured concentrations to normal conditions, resulting in unreliable results.
Here is an excerpt from the Standard:
"Measurements lead to standard conditions using the following formula:
C 0 = C 1 * P 0 T 1 / P 1 T 0
where: C 0 - the result expressed in terms of mass per unit volume of air, kg / cu. m, or the amount of matter per unit volume of air, mole / cube. m, at standard temperature and pressure;
C 1 - the result, expressed in terms of mass per unit volume of air, kg / cu. m, or the amount of matter per unit volume
air, mol / cu. m, at temperature T 1, K, and pressure P 1, kPa. "
The formula for reducing to normal conditions in a simplified form has the form (2)
C 1 = C 0 * f, where f = P 1 T 0 / P 0 T 1
standard recalculation coefficient of reduction to normal conditions. Air and impurity parameters are measured at different values of temperature, pressure and humidity. The results lead to standard conditions for comparing the measured air quality parameters in different places and different climatic conditions.
3.2. Sectoral Normal Conditions
Normal conditions are standard physical conditions, which are usually related to the properties of substances (Standard temperature and pressure, STP). Normal conditions are defined by IUPAC (International Union of Practical and Applied Chemistry) as follows: Atmospheric pressure 101325 Pa = 760 mm Hg. Air temperature 273.15 K = 0 ° C.
Standard conditions (Standard Ambient Temperature and Pressure, SATP) are normal ambient temperatures and pressures: pressure 1 bar = 10 5 Pa = 750.06 mm Tg; temperature 298.15 K = 25 ° C.
Other areas.
Measurement of air quality.
The results of measurements of concentrations of harmful substances in the air of the working zone lead to the following conditions: temperature 293 K (20 ° C) and pressure 101.3 kPa (760 mm Hg).
Aerodynamic parameters of emissions of pollutants should be measured in accordance with applicable state standards. The volumes of waste gases obtained from the results of instrumental measurements should be reduced to normal conditions (nos): 0 ° C, 101.3 kPa ..
Aviation.
The International Civil Aviation Organization (ICAO) defines an international standard atmosphere (International Standard Atmosphere, ISA) at sea level with a temperature of 15 ° C, an atmospheric pressure of 101325 Pa and a relative humidity of 0%. These parameters are used in the calculation of the movement of aircraft.
Gas economy.
The gas industry of the Russian Federation, when calculating with consumers, uses atmospheric conditions in accordance with GOST 2939-63: temperature 20 ° С (293.15 К); pressure 760 mm Hg. Art. (101325 N / m²); the humidity is 0. Thus, the mass of cubic meter of gas in accordance with GOST 2939-63 is slightly less than under "chemical" normal conditions.
Testing
For the testing of machines, instruments and other technical products for normal values of climatic factors in the testing of products (normal climatic test conditions) take the following:
Temperature - plus 25 ° ± 10 ° C; Relative humidity - 45-80%
Atmospheric pressure of 84-106 kPa (630-800 mm Hg)
Verification of measuring instruments
The nominal values of the most common normal influencing variables are selected as follows: Temperature - 293 K (20 ° C), atmospheric pressure - 101.3 kPa (760 mm Hg).
Rationing
In the guidelines on the establishment of air quality standards, it is indicated that MPCs in ambient air are installed under normal indoor conditions, i.e. 20 C and 760 mm. gt; Art.
7. International norms and rules in the field of atmospheric air protection. International standardization of air quality to ensure uniformity of measurements.
To date, there is no single international rules and regulations in the field of atmospheric air protection. The air quality is normalized depending on the specifics of the country. Many countries use the World Health Organization (WHO) air quality standards to develop their standards, which were developed and published in 1999 and recommended for use in the assessment of disease risks. Many countries base their standards on national air quality standards for the United States (primarily the countries of the Americas, Australia and New Zealand), as well as the standards of the EU countries (Europe and North Africa). From what has been said, it follows that international standardization of air quality requirements is needed, as well as standardization of control methods in both interstate and public organizations. The leading international organization is ISO, the international organization for standardization, whose main task is the development and publication of international standards, including in the field of environmental protection. In order to develop standards in the field of air quality, the Technical Committee ISO TK 146 "Air Quality" was established.
- Concentration conversion formulas are given that relate the relative concentrations of polluting chemicals Cw and the absolute mass concentrations CxxV with the meteorological parameters T and P at the measurement site.
- Since the measured values of the gas concentrations for the meteorological parameters at the measurement point are related to their values under normal conditions T 0, P 0 by a simple conversion formula, it is necessary to correct the measured values of the GC concentrations to normal conditions. To this end, procedures were provided for registering meteorological parameters in air quality measurement protocols. This complicated and increased the cost of processing data and the accumulation of statistical material.
- The resulting concentration expression 1.4 with the reduction formula (4) can be called ppm Tp, thereby emphasizing the fact that the temperature correction T and the pressure P were corrected at the time of measurement in order to bring the measured values of the ZHB concentration to normal (standard conditions) in a single measuring module without resorting to to a separate recounting procedure.
- The last definition of 1.5 ppm is determined by formula (5), it seems most preferable, since the concentration measured by the instrument under observation conditions does not need to be reduced to normal conditions, since it coincides with it, but its use will lead to a correction of GOST.
- The parameters of the normal measurement conditions have a clearly expressed sectoral and specialized nature, and this fact obliges to normalize the measured parameters of air pollution in accordance with the requirements of industry standards.
- To ensure the uniformity of measurements and control of the quality of the environment, ISO, WHO, IUPAC and other international organizations need to develop unified standards in the ambient air that are similar to the standards of individual states, for example, in the Russian Federation GOST 8.417-2002 (Units of physical quantities).
- It is necessary to unite efforts for creation of uniform norms for an estimation of quality of air, tk. WHO standards are recommendatory.
- Quality of air. General provisions. Units of quantities. M., 2013
- GOST 8.417-2002 GSI. Units of physical quantities - M., 2010
- Standard of the Russian Federation. AIR QUALITY GOST R ISO 8756-2005 PROCESSING OF DATA ON TEMPERATURE, PRESSURE AND HUMIDITY.-M., 2007
- GOST 8.578-2008 State system for ensuring the uniformity of measurements STATE CIRCUIT SCHEME FOR MEANS OF MEASUREMENT OF CONTENT COMPONENTS IN GAS MEDIA-M., 2009
- The EU-Russia report. Harmonization of environmental standards. Activity block 10. 10.3 Normalization of air quality and emissions of pollutants. - M., 2008
- GOST 12.1.005-88 GENERAL SANITARY-HYGIENIC REQUIREMENTS TO AIR OF THE WORKING ZONE. - M., 1988, 2006
- METHODOLOGICAL MANUAL FOR CALCULATION, NORMALIZATION AND MONITORING OF EMISSIONS OF POLLUTANTS IN ATMOSPHERIC AIR. - St. Petersburg, 2012.
- GOST 15150-69 MACHINES, INSTRUMENTS AND OTHER TECHNICAL EQUIPMENT. Versions for different climatic regions. - M., 2006
- GOST 8.395-80 NORMAL CONDITIONS OF MEASUREMENTS UNDER THE VERIFICATION .- M., 2001
- Temporary methodological guidelines for justifying the maximum allowable concentrations (MPC) of pollutants in the atmospheric air of populated areas MU 4681-88 - M., 1989
- REQUIREMENTS FOR THE STATEMENT OF EXPERIMENTAL RESEARCHES ON THE RATIONALE OF MAXIMUM ALLOWABLE CONCENTRATIONS OF INDUSTRIAL CHEMICAL ALLERGENS IN THE AIR OF THE WORKING ZONE AND ATMOSPHERES METHODICAL INSTRUCTIONS MU 1.1.578-96 - M., 1997
- Guidelines for air quality. WHO 2000,
- National emission standards for hazardous air pollutants.
Calculate the minimum air volume needed to determine the bromine vapor, if the sensitivity of the method is 0.002 mg, the maximum permissible one-time concentration of bromine vapor in the production plant is 0.5 mg / m 3 or 0.0005 mg / l. The total volume of the absorbing solution is 4 ml, and for analysis 2 ml is taken. The coefficient expressing the part of the MPC to be determined is 0.5.
The air temperature at the time of sampling 20 0 C, atmospheric pressure 750 mm Hg. Art.
Calculation of the required air volume is carried out according to the formula:
V0 =,
where: a - the sensitivity of the determination method (the minimum detectable amount of substance in milligrams);
V is the total volume of the absorbing solution, ml;
K is the total coefficient that displays the part of the maximum permissible concentration that is to be determined (1, 1/3, etc.);
C 0 is the maximum permissible concentration of the substance, mg / m 3;
V 1 - volume of the absorbing solution, taken for analysis, ml.
Solution: V 0 = 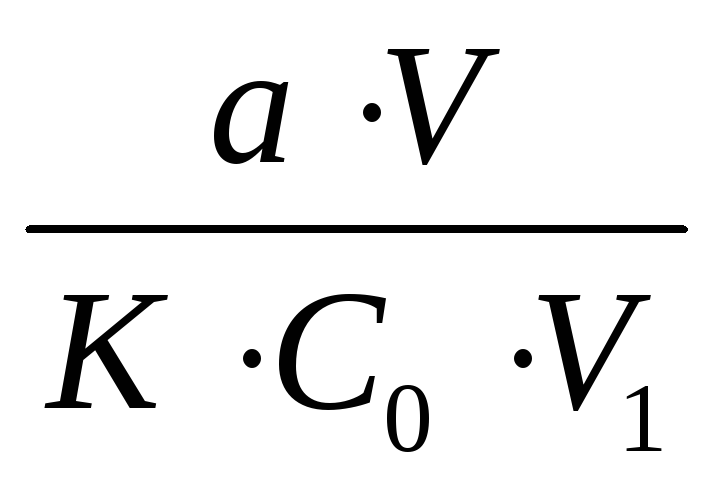 =
=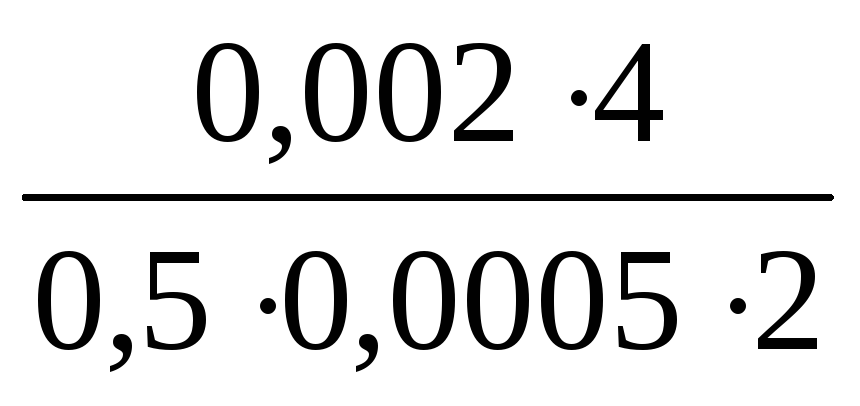 =
=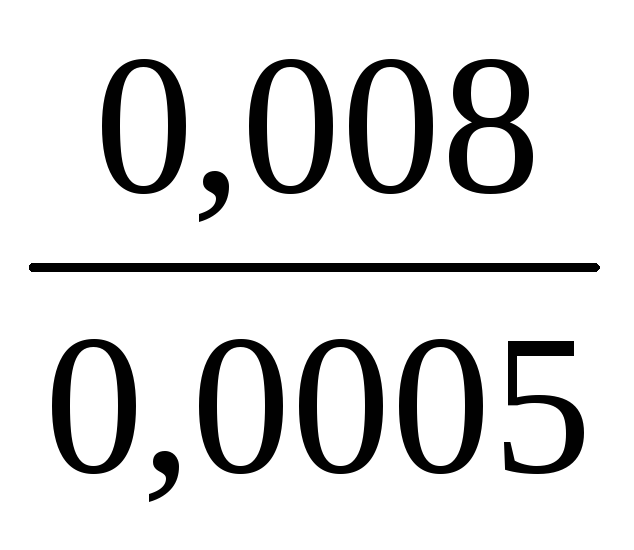 = 16 liters.
= 16 liters.
The reduction of the volume of air taken in liters to normal conditions is carried out according to the Gay-Lussac formula:
V0 = Vt ,
where V 0 is the volume of air reduced to normal conditions (0 0 С and 760 mm Hg), л;
V t is the volume of air sampled for analysis at a given temperature and barometric pressure, l;
273 - coefficient of gas expansion;
T - air temperature during sampling, 0 С;
B - atmospheric pressure during sampling, mm Hg.
In order to facilitate calculations, the importance of the constituents of formula 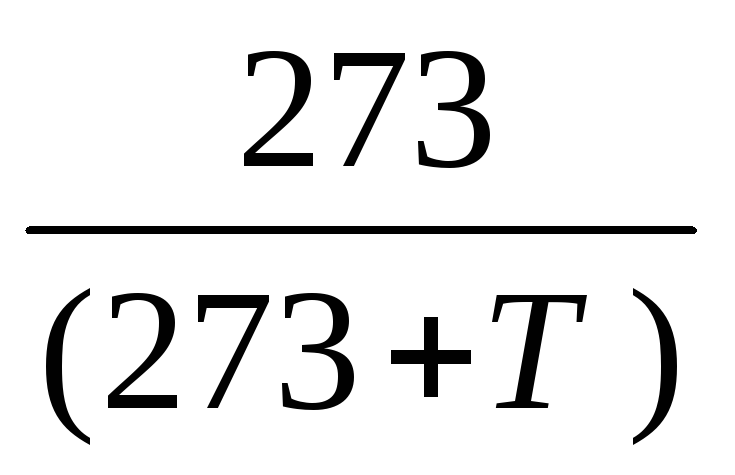 and
and 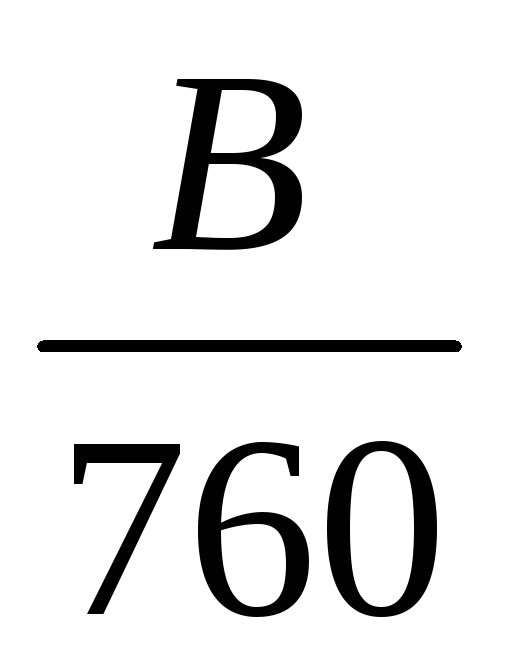 are found in Table 1.
are found in Table 1.
Vo = Vt = 16 л = 16 0.932 0.987 = 14.72 l
Table 1.
Coefficients to bring air volumes to normal conditions.
|
Temperature |
Barometric pressure, |
Temperature |
Barometric pressure, mm Hg. | ||||