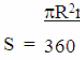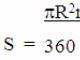Mining, varieties and use of diamonds. Using diamonds What can be made from diamond
In this article:
If we talk about precious stones, then the most versatile of them and at the same time the most expensive are diamonds. This is confirmed by what is made of diamonds today, since stones are used not only in the jewelry industry, but also in industry. Minerals are even classified by use: into industrial and precious specimens. At the same time, industrial diamonds can be both natural and synthetic.
Diamond quality
Diamond has good physical and chemical properties. The mineral has a unique crystal lattice structure, which is the most compact in nature. It is this factor that ensures the maximum hardness of the substance. And the stone is fragile, which allows it to be turned into diamond dust.
Rings with diamonds
The density of the stone is within the following indicators: 3.4-3.5 g / cm3. But the thermal conductivity of diamond is high, which is not typical for other precious stones. Important characteristics are low compression ratio and high modulus of elasticity.
The mineral has a high melting point - 4000 degrees Celsius, and if the process is carried out without access to oxygen, the stone will melt at a temperature of 2000 degrees and turn into graphite. After this, the changes that occur to the diamond are anomalous and defy classification. Therefore, there can be no doubt about the unique properties of this stone.
In jewelry, a stone is turned into a diamond. The process of processing a diamond is a painstaking task that requires precision, it takes place in several stages:
- sawing the copy into pieces;
- shaping the stone
- diamond cut.
Every detail in the production is important, because the result depends on the professionalism of the master. You can spoil a diamond, or, on the contrary, you can give it an ideal shape, hide flaws and defects with the help of cutting by a specialist. Processing is carried out at special factories and large firms that buy raw materials. Jewelry still holds the leading position in terms of diamond consumption.
Before proceeding to the third stage of cutting, you need to think over and draw a diagram of the finished stone with all sizes. The cost of the stone depends on the accuracy of the cut and the size of the girdle, crown and pavilion. The finished diamond goes through the evaluation stage according to four parameters:
- the purity of the stone, the presence or absence of defects;
- faceting - varieties of form, as well as the economy of the process (waste minimization);
- weight - a parameter that is measured in carats;
- color - from a bluish to yellowish shade.
In the future, the stone is sold or set into a product. The cost of the stone increases with each subsequent stage of processing, and as a result, the price of a diamond increases by at least three times. A diamond will adorn any precious metal and any kind of product: earrings, pendants, bracelets, rings. The size and characteristics should be selected based on the cost of the product. And also do not forget to check all certificates and other documents confirming the authenticity of the stone.
Industrial use
The industrial use of diamonds is also widespread due to their properties. In such cases, natural stones are used that are mined in nature and have flaws that do not allow cutting. They also use minerals obtained in laboratories - the so-called synthetic diamonds. At the same time, the cost of diamonds is much cheaper due to the low cost of raw materials.

Artificial diamonds in industry
It is beneficial to use a mineral, since tools that have a diamond coating do a better job - there are no microcracks on the materials. The mineral copes with such tasks as sawing concrete, crushing rubble, cutting granite and marble. That is, even solid substances lend themselves to it, since the mineral is the hardest substance on Earth.
And diamonds in one form or another are used:
- In turning metal, since the hardness of the diamond will allow the process to be carried out neatly and without flaws.
- When coating tools: glass cutters, saws, drills, milling cutters. This is necessary in order to increase the durability of devices and ensure the accuracy of their work.
- In medicine, in particular, the manufacture of surgical instruments (scalpels, clamps, scissors), since such instruments accurately perform all incisions and incisions and at the same time remain sharp for a long time.
- In telecommunications, the mineral is used to make cables, since it retains and allows the signal to be transmitted over long distances, despite temperature and voltage fluctuations. Diamond dies help achieve the perfect cut.
- Use in space shipbuilding, optics and laser technologies.
- During the construction of tunnels, as well as where there is an explosive atmosphere.
- As detectors of nuclear radiation.
- In the work of oil companies, as well as in the extraction of other minerals, where drills, drills and other equipment are used.
The use of diamond occurs in different forms, but diamond chips are most often used. This is a cheap substance, but it is available only to manufacturers. In the industry, such forms are popular as.
We are glad to welcome you once again, our dear readers! Diamonds have always been different from other minerals. And not only by the fact that beautiful and cut diamonds are obtained from them, but also by their widest and most diverse applications in industry, dentistry, laser medicine and other industries. The properties of diamond allow you to do all this.
We will cover them in this short, informative and, we are sure, interesting article. It should be noted that some of the properties of this stone can be used at home, thereby finding a way out of unusual and non-standard life situations.
Let's start studying such an interesting topic. We wish you a pleasant reading, our dear friends!
Physical properties of diamond
Let's start with the most famous, namely the physical properties, since it was they who allowed this stone to gain such popularity. Consider the following "professional" qualities:
Mineral hardness
Almost everyone knows that a mineral like diamond is the hardest known stone in the world. What is the reason for this? The specific crystal lattice of the mineral. The bonds between carbon atoms are very strong.

To assess the relative values of the hardness of minerals, there is the Mohs scale, which is known and accepted throughout the world. Relativity (we will explain as easily as possible) was taken as the basis of the following: the scratching of one mineral relative to other reference ones. For example, a diamond piece can "scratch" all the minerals, but almost nothing. That's the whole principle that helps to significantly simplify life.
Diamond shards lead there by a solid margin, with a score of 10. For example, the closest thing to the hardest mineral on Earth is corundum. It was also evaluated on this scale and has a score of 9. That is, its value is 150 times less!
Only on the basis of these numbers can one imagine the significant advantage of the hardest known mineral. One of the clearest examples is cutting glass with a diamond-tipped glass cutter. One has only to draw a straight line with a non-shaking hand, slightly press on the other end of the glass - and you're done. With the help of other elements and minerals, this is difficult to achieve.

It should also be noted the use of diamond hardness when digging and digging mines, underground recesses, new metro lines and underwater channels using a special installation, the tips of which are made of diamonds and allow cutting through even the most complex iron-granite rock.
Although this unit is expensive, it pays off compared to payments to workers who would do the same amount. Especially in terms of time characteristics, the installation wins significantly. If you haven't yet imagined how it might look and work, then you can read the writer Jules Verne or watch the 2005 film Expedition to the Underworld.
Density, refractive index and dispersion characteristic of the stone
- The unique construction of the crystal lattice explains its high density, which also finds application in various fields. Hardness and density are closely related to each other. The higher one parameter, the higher, as a rule, the second.
- The refractive index and dispersion are most pronounced in diamonds - faceted diamonds. It is in them that you can see the amazing magic and play of light, indescribable brilliance, which will delight connoisseurs.
The diamond is so unique that the rays of light passing through it pass almost perfectly according to optical laws, and the high refractive index provides "internal brightness" and an even greater light play of the stone. For greater clarity and understanding, the picture below will explain to you much better what is described in words:

The characteristic, of course, also found its application in the most famous business for diamonds - jewelry, where the most stunning and best diamond and diamond specimens mined from the bowels of our planet Earth are collected.
The unique characteristic of the stone is thermal conductivity
- The thermal conductivity of diamond is the highest among known solids and is on the order of 0.9-2.3 kW/(m*K). As a result, diamond is an excellent semiconductor, as the best-known silicon semiconductor elements generally operate at temperatures around 100 degrees Celsius.
Semiconductor technology based on diamond elements allows operation at much higher temperatures, but given the high cost, this is often an unjustified luxury. There is also an expedient replacement for them - synthetic diamond semiconductor elements that have the same high thermal conductivity as natural stones, but cost much less.

Other significant properties
- In addition to the above properties, a diamond has a lot of other, no less significant and useful criteria. One of these properties is that diamond is a dielectric. This mineral does not conduct electricity.
This property is especially important in electronics, semiconductor, medical and laser technology. This feature allows you to simultaneously not conduct electricity (thus not cause a short circuit and breakdown in the system) and transmit a large flow of powerful energy (for example, laser systems) without losing either their qualities, their characteristics, or their weight. Another unique feature of the diamond.
- It is also worth noting an important quality for industry - a low coefficient of friction for metal in the presence of air.
This is due to the formation of a thin film when exposed to heat. This film plays the role of a special material lubricating two surfaces. Have you noticed special diamond blades designed for a tool that can cut concrete slabs and bases, thick-walled metal and at the same time serve for a long time in construction stores? Here, please, you have a visual application of this property, which greatly simplifies life.

- High melting point (about 3700-4000 degrees Celsius at an ambient pressure of 11 GPascals). Under normal conditions, the diamond begins to burn only somewhere at 820-860 degrees Celsius.
Such a peculiar and amazing property also finds its application, for example, in those spare parts or elements of equipment that are constantly exposed to such temperatures and where their use is justified in comparison with the price and payback period.
If we combine all the above properties of diamonds, we can conclude that regarding the physical properties of diamonds, the value of the stone is enormous, both in the field of jewelry and in various fields of industry, electronics, and optics.
The magical properties of diamond
Since the most ancient times, it was believed that such a unique stone should simply have supernatural powers. Suffice it to recall the magic skulls made of crystal and diamonds of the ancient and suddenly disappeared Mayan people, the era of the pharaohs, where all the kings and queens were simply “studded” with diamonds and expensive jewelry from them.
Diamond has always been considered the stone of strong people. This stone, according to many beliefs, gives strength, courage, valor and courage. No wonder it is called the "stone of kings." It is also believed that this is a strong amulet that allows the owner to avoid negative influences from third parties.

It should be noted that in ancient times, the magical properties of a diamond could neutralize any drink from poison. It was enough just to lower the stone there and wait a few minutes. (We do not recommend checking this).
Also, the magical properties of a diamond are known in the love sphere of Cupid. In the same Ancient Egypt, it was believed that if you hold a stone on your fingertips or take diamond powder, then such a rite promises boundless and reciprocal love until the last day.
A diamond is a stone that directly reflects the biofield of a person's owner. If it is good, then the stone will contribute to the emergence and preservation of money, luck, love, strength and other positive manifestations. Also, the stone will protect against envious people and bad actions directed against the owner.
In the case of bad karma, it is usually the other way around. But there may also be a possibility that the diamond will “pull out” bad energy and allow a person to be “reborn”.
For the best effect, wear the diamond stone so that it touches the skin. For example, on the neck as a pendant or on the left hand as a bracelet.
Also, there are three more things to consider:
- as a rule, a diamond is given to a person, and not bought for oneself. This shows recognition and honor to a person, which is taken for granted by a diamond;
- the more people in contact with the stone, the better, since it can affect not only the person himself, but also his work, personal life, family atmosphere.
- pay special attention to the color before buying. Red refers to the passionate and harsh elements of fire, blue - watery calm, white - neutral.
In the end, you can talk a little about the influence of the stone and the signs of the zodiac. Since the stone is strong, only strong and powerful signs, for example, signs of the fire element, can own it.
But people born under the sign of fish should try to stay away from him, as he can even cause a negative impact. You should also pay attention to the same color shade of the diamond or diamond.
Healing magic of the diamond
The large energy potential of the stone is able to charge the cells of the human body with positive energy and help it cope with various kinds of negative diseases.
Diamond has a special effect on the mental and psychological state of the brain, as well as on the regulation of normal biorhythms and the well-functioning work of the cardiovascular system.

Team LyubiKamni
Hello dear guests and readers. For a very long time, diamonds have been valued as one of the hardest minerals on our planet. But these stones began to be used as an industrial material not so long ago - less than a century has passed since that time. Many experiments have been carried out to determine the qualities of a diamond, which you can read about here.
You may have heard that a diamond can only be cut with... another diamond. Unfortunately, no other materials are suitable for such a delicate process. This prompted scientists to conduct a variety of experiments in order to fully reveal such many-sided qualities of the mineral.
Features of use
- Imitation of natural conditions, as close as possible to real events. For this, explosives with carbon are used.
- Chemical deposition of diamonds from vapor (rather laborious process).
- pressure and temperature. Their values must be high enough for such a beautiful stone to be born.
In addition, during the extraction of diamonds, a large proportion of them are rejected as unsuitable for the manufacture of diamonds. The reasons may be various kinds of cracks and chips, cloudy color, etc. Such diamonds are usually called technical, since they find their application there.
Thus, at the moment, the industry fully provides itself with the necessary amount of the hardest mineral. And perhaps it became only in the twentieth century.
It is even difficult to imagine how modern technologies would develop if they did not have such an assistant as this stone.
Team LyubiKamni
Diamond- the hardest mineral, cubic polymorphic (allotropic) modification of carbon (C), stable at high pressure. At atmospheric pressure and room temperature, it is metastable, but it can exist indefinitely without turning into graphite, which is stable under these conditions. In a vacuum or in an inert gas at elevated temperatures, it gradually transforms into graphite.
STRUCTURE
The crystal system of diamond is cubic, space group Fd3m. The unit cell of the diamond crystal lattice is a face-centered cube, in which carbon atoms are located in four sectors arranged in a checkerboard pattern. Otherwise, the diamond structure can be represented as two cubic face-centered lattices, offset relative to each other along the main diagonal of the cube by a quarter of its length. A structure similar to diamond has been established for silicon, a low-temperature modification of tin, and some other simple substances.
Diamond crystals always contain various defects in the crystal structure (point and line defects, inclusions, subgrain boundaries, etc.). Such defects largely determine the physical properties of crystals.
PROPERTIES

Diamond can be colorless, translucent or colored in various shades of yellow, brown, red, blue, green, black, gray.
The color distribution is often uneven, patchy or zonal. Under the action of X-ray, cathode, and ultraviolet rays, most diamonds begin to glow (luminesce) in blue, green, pink, and other colors. It is characterized by exceptionally high light refraction. The refractive index (from 2.417 to 2.421) and strong dispersion (0.0574) determine the bright brilliance and multi-colored "play" of faceted gem diamonds, called brilliants. Shine is strong, from diamond to greasy. Density is 3.5 g/cm 3 . According to the Mohs scale, the relative hardness of diamond is 10, and the absolute hardness is 1000 times higher than the hardness of quartz and 150 times that of corundum. It is the highest among all natural and artificial materials. However, it is quite fragile and breaks easily. The fracture is conchoidal. It does not interact with acids and alkalis in the absence of oxidizing agents.
In air, the diamond burns at 850 ° C with the formation of CO 2; in vacuum at temperatures above 1.500 ° C, it transforms into graphite.
MORPHOLOGY
The morphology of diamond is very diverse. It occurs both in the form of single crystals and in the form of polycrystalline intergrowths (“board”, “ballas”, “carbonado”). Diamonds from kimberlite deposits have only one common flat-faced shape - an octahedron. At the same time, diamonds with characteristic curved shapes are common in all deposits - rhombic dodecahedroids (crystals similar to a rhombic dodecahedron, but with rounded faces), and cuboids (crystals with a curvilinear shape). As shown by experimental studies and the study of natural samples, in most cases, crystals in the form of a dodecahedroid arise as a result of the dissolution of diamonds by a kimberlite melt. Cuboids are formed as a result of the specific fibrous growth of diamonds according to the normal growth mechanism.

Synthetic crystals grown at high pressures and temperatures often have cube faces and this is one of the characteristic differences from natural crystals. When grown under metastable conditions, diamond easily crystallizes in the form of films and columnar aggregates.
The sizes of crystals vary from microscopic to very large, the mass of the largest Cullinan diamond, found in 1905. in South Africa 3106 carats (0.621 kg).
Several months were spent studying the huge diamond, and in 1908 it was split into 9 large pieces.
Diamonds weighing more than 15 carats are rare, and those weighing over a hundred carats are unique and are considered rarities. Such stones are very rare and often get their own names, world fame and their own special place in history.
ORIGIN
Although diamond is metastable under normal conditions, due to the stability of its crystal structure, it can exist indefinitely without turning into a stable carbon modification - graphite. Diamonds brought to the surface by kimberilites or lamproites crystallize in the mantle at a depth of 200 km. and more at a pressure of more than 4 GPa and a temperature of 1000 - 1300 ° C. In some deposits, deeper diamonds are also found, taken out of the transition zone or from the lower mantle. Along with this, they are brought to the Earth's surface as a result of explosive processes accompanying the formation of kimberlite pipes, 15-20% of which contain diamond.
Diamonds are also found in ultrahigh pressure metamorphic complexes. They are associated with eclogites and deeply metamorphosed garnet gneisses. Small diamonds have been found in significant quantities in meteorites. They are of very ancient, pre-solar origin. They also form in large astroblems - giant meteorite craters, where remelted rocks contain significant amounts of fine-grained diamond. A well-known deposit of this type is the Popigai astrobleme in northern Siberia.

Diamonds are rare, but at the same time quite widespread mineral. Industrial diamond deposits are known on all continents except Antarctica. Several types of diamond deposits are known. For several thousand years, diamonds have been mined from alluvial deposits. Only towards the end of the 19th century, when diamond-bearing kimberlite pipes were first discovered, did it become clear that diamonds were not formed in river sediments. In addition, diamonds have been found in crustal rocks in associations of ultrahigh pressure metamorphism, for example, in the Kokchetav massif in Kazakhstan.
Both impact and metamorphic diamonds sometimes form very large-scale deposits, with large reserves and high concentrations. But in these types of deposits, the diamonds are so small that they have no industrial value. Commercial diamond deposits are associated with kimberlite and lamproite pipes confined to ancient cratons. The main deposits of this type are known in Africa, Russia, Australia and Canada.
APPLICATION
Good crystals are cut and used in jewelry. About 15% of mined diamonds are considered jewelry, another 45% are considered near-jewelry, that is, inferior to jewelry in size, color or clarity. Currently, the global diamond production is about 130 million carats per year.
Diamond(from the French brillant - brilliant), - a diamond, which, through mechanical processing (cutting), is given a special shape, a brilliant cut, which maximizes such optical properties of the stone as brilliance and color dispersion.
Very small diamonds and fragments, unsuitable for cutting, are used as an abrasive for the manufacture of diamond tools necessary for processing hard materials and cutting diamonds themselves. A cryptocrystalline variety of black or dark gray diamond that forms dense or porous aggregates is called Carbonado, has a higher abrasion resistance than diamond crystals and is therefore especially valued in industry.
Small crystals are also grown artificially in large quantities. Synthetic diamonds are obtained from various carbon-containing substances, mainly from graphite, in special. apparatuses at 1200-1600°C and pressures of 4.5-8.0 GPa in the presence of Fe, Co, Cr, Mn or their alloys. They are suitable for technical use only.
Diamond - C
CLASSIFICATION
| Strunz (8th Edition) | 1/B.02-40 |
| Dana (7th edition) | 1.3.5.1 |
| Dana (8th edition) | 1.3.6.1 |
| Hey's CIM Ref. | 1.24 |
PHYSICAL PROPERTIES
| Mineral color | colorless, yellowish brown fading to yellow, brown, black, blue, green or red, pink, cognac brown, sky blue, lilac (very rare) |
| Dash color | no |
| Transparency | transparent, translucent, opaque |
| Shine | diamond, bold |
| Cleavage | octahedral perfect |
| Hardness (Mohs scale) | 10 |
| kink | uneven |
| Strength | fragile |
| Density (measured) | 3.5 - 3.53 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Thermal properties | High thermal conductivity. Feels cold to the touch, which is why a diamond is called "ice" in slang |
Diamond (from Arabic ألماس, ’almās, Tur. elmas, which goes through Arabic from other Greek ἀδάμας - “indestructible”) is a mineral from the class of native elements, one of the allotropic modifications of carbon. Chemical formula: S.
Diamond has the same chemical composition as graphite. But in appearance it differs sharply from it. This difference is explained by the different arrangement of carbon atoms in the crystal lattice: in diamond they are placed in a tetrahedral structure and have a strong bond in all directions. Specific gravity 3.48-3.55 g/cm 3 . Diamond is a stone with unusual brilliance, play of colors, inner fire. The brilliance of a diamond is strong - diamond. Diamond is very hard - "the king of all minerals."
On the Mohs scale, it has a hardness of 10. In terms of hardness, it is not inferior to any of the known minerals. Diamond is the "champion of hardness": it is 1000 times harder than quartz, 150 times harder than corundum. Maybe that's why the ancient Greeks considered the diamond a talisman of power. Diamond is resistant to acids, heat. It is the only mineral that scratches corundum. On this basis, it differs from minerals similar to it - rock crystal, topaz, etc. Diamond is very hard, but at the same time fragile. It easily splits along the cleavage planes. Cleavage is perfect but to the faces of the octahedron. This property of a diamond is used by jewelers when processing it. A new mineral with great hardness has been found, the "brother" of diamond - yakutite.
No gem has as many shades as a diamond: colorless, white, blue, green, yellowish, pink, reddish, brownish, smoky gray tones; often transparent.
Diamond is found mostly in the form of individual crystals - octahedrons with curved edges, in external shape approaching a ball. Crystal sizes are usually small. Crystallizes in the cubic syngony.
Features. Characteristic features for a diamond are a strong diamond splash and high hardness - it leaves a scratch on corundum. If metallic aluminum is used to draw on the wetted surface of a diamond, the aluminum leaves no traces.
Varieties and photos of diamond
- Diamond- an artificially processed diamond with 57 facets. A diamond scatters sunlight like raindrops forming a rainbow, a diamond is the most radiant gemstone.
- Board- Irregular fine-grained splices.
- Ballas- spherical diamond, radial-radiant structure.
- Carbonado- black, gray, dense or coarse-grained diamond.
- Yakutit- dark diamond with numerous inclusions and maximum hardness.
Colorless diamond, Catoca, Angola Bort Spherical ballas Black carbonado
Origin of diamond
Diamond deposits are genetically related to ultrabasic (dunites, peridotites) and basic (diabases) igneous rocks and to serpentinites resulting from chemical alteration of ultrabasic and basic rocks. Diamond is formed under conditions of high pressure and high temperature, so its deposits are confined to volcanic explosion craters. Diamond is formed at a pressure of more than 5 MPa and a temperature of about 2000 ° C.
The formation of diamonds is closely related to tectonic processes. At the same time, a fiery-liquid mass, the so-called ultrabasic magma, rose from great depths along those that arose in the earth's crust. It is sometimes called kimberlite. As the kimberlite magma ascended, it cooled and this led to the separation of dissolved volatile compounds (gases, water vapor). The released water vapor and gases caused strong explosions, as a result of which vertical well-shaped cylindrical holes appeared in the earth's crust - kimberlite pipes. These tubes were filled with crushed rocks formed during the explosion. Then, along a funnel filled with clastic material, kimberlite magma rose, which occupied the voids between the debris and cemented them.
The diamonds are thought to have erupted mostly in solid form when the kimberlite magma was still at depth, and then they were carried by the magma flow into the kimberlite pipes. Diamonds contain only those pipes whose roots reach the diamondiferous layer. Diamonds are formed at depths of about 200 km.
Findings of diamonds are known not only on platforms (on the plains), but also in mountainous areas: in the Urals, in the Appalachians, the Cascade Mountains, the Sierra Nevada, on about. Kalimantan and other areas.
Diamonds are found in meteorites. Diamond is also formed during explosions that accompany the fall of huge meteorites (Meteoritic Crater "Devil's Canyon", Arizona, USA).
It occurs among basic and ultrabasic igneous rocks, among serpentinites (serpentines); also in ancient (conglomerates, sandstones) and young placers.
satellites. In primary deposits: serpentine, olivine, augite, graphite, magnetite, chromite, ilmenite, talc. In placers: quartz, platinum, gold, magnetite, ilmenite, hematite], topaz, cassiterite, corundum. A constant companion of diamond is pyrope, a cherry-colored mineral. Pyrope is more common than diamond, and serves as a good "landmark" when looking for diamond deposits.
Application of diamond
Diamonds are divided into jewelry and technical. The former include transparent, colorless or slightly colored varieties of more or less large sizes; to technical ones - dark marginal differences and small-sized diamonds. In the deposits, as a rule, technical diamonds predominate, gem grades are less common.
Diamond is called the hero of technology. Up to 80% of diamonds mined worldwide are used in industry. Diamonds are used in the electrical, radio-electronic and instrument-making industries. Diamonds are used as nuclear radiation detectors, in fast particle counters, and in medical counters. They are used in space research, in the study of the deep structure of the Earth. The use of diamond for cutting glass is well known. A 1-carat diamond (a carat equals 0.2 g) can cut window glass 2,500 km long.
A diamond, comparable to the transparency of spring water, shimmers with all the colors of the rainbow and is also used as jewelry (diamond). It is valued more than a hammer. For the cost of a diamond the size of an apricot, you can build an entire factory. The high price of a diamond is explained not so much by its high hardness, strong brilliance, beautiful “play” of colors, but by its rarity. Large deposits are rare. Even in rich deposits, 3-6 small diamond grains are found in one cubic meter of rock.
On average, only about 5 kg of diamonds are extracted from 100,000 tons of rock. The ratio is 20 million to 1.
The history of the diamond has more than five thousand years. Eminent diamonds and other precious stones are witnesses of power, immense splendor of royal outfits, people's grief, suffering. Diamonds adorned crowns and other attributes of power of pharaohs, shahs and kings.
Many of the big diamonds have bloody histories full of secrets, tragedies, nightmarish crimes, applied by fleeting greedy joy in the world of gain.
Diamond deposits
Africa is the Diamond Continent. The main diamond-producing countries on African soil are: the Republic of Zaire, which ranks first in the world in the extraction of industrial diamonds, Tanzania, Ghana, South Africa (the country of diamonds is Namibia, which ranks first in the world in the extraction of gem diamonds, illegally occupied by South Africa), Angola, Guinea and other. One of the richest in Africa and in the world are the diamond deposits of the Central African Empire. Then come the countries of South America: Brazil, Venezuela, Guyana and Asian countries: India, Indonesia.
In South Africa in 1905, two giant diamonds were found. The largest of them is "Cullinan" (named after the owner of the mine) weighing 3106 carats (the size of a fist), the second - "Excelsior" - 971.5 carats. Both diamonds were sawn and cut into smaller diamonds and sold. The Cullinan yielded 105 diamonds after sawing. Two of them - the largest - are inserted into the royal scepter and the imperial crown of England. In Sierra Leone, in the Enge-ma region (West Africa), a large diamond was found the size of a small chicken egg. It weighs 968.9 carats (almost 200 g). Its length is 40 mm. They called it the "Star of Sierra Leone". In the international list of rare diamonds, it ranks third. Diamond "Star of Sierra Leone" cut into 11 individual stones of a high price. The quality of Sierra Leonean diamonds is one of the best. The largest Indian diamond "Great Mogul" - 794 carats. Large diamonds "Orlov" (194.8 carats) and "Koh-i-nur" (109 carats) were found in India.
The largest flat diamond has an area of 7.5 cm2. It is mounted in a gold bracelet; stored in the diamond fund of Russia. One of the largest light blue diamonds of 42.27 carats was found in the Republic of South Africa (Orange Province).
The very first diamond in Russia was found by 14-year-old serf Pavel Popov in the Urals in the 19th century. After such a precious find, geologists explored the Urals and Siberia for almost 100 years, until the geologist Larisa Popugaeva in June 1954 found the first Zarnitsa kimberlite pipe in cold Yakutia. The name of Larisa Popugaeva bears one diamond weighing 29.4 carats.
The Yakut diamond is clean and transparent, as if the fortress of the Yakut frost has absorbed the beauty of the northern lights. About ten kimberlite pipes have been discovered on the territory of Yakutia: Aikhal, Zarnitsa, International, Mir, the world's largest Udachnaya, Yubileinaya. One of the large Soviet diamonds "Maria" weighs 105.98 carats. A diamond weighing 342.5 carats was found in the Mir pipe on December 23, 1980 and named after the 17th Congress of the CPSU, which took place 3 months after the discovery. In modern Russia, two finds stand out, made in 2003 in the Udachnaya pipe: lemon-colored and tobacco-colored diamonds, weighing 301.55 and 232.7 carats, respectively.
Kimberlite pipes and associated diamond deposits are found in Russia not only in Yakutia. The discovery of diamond deposits here was the discovery of the Pomorskaya kimberlite pipe in 1980, which, in addition to another 5 pipes (Pionerskaya, Karpinsky-1 ″, Karpinsky-2, Arkhangelskaya and Lomonosov) is part of the largest placer diamond deposit in the European part of Russia - named after M.V. Lomonosov. Here, the largest in the history of the development of the deposit is a diamond weighing 50.1 carats. In the Arkhangelsk region, in addition to the Lomonosov deposit, the V.P. Mushroom (Verkhotinskoe).
One of the promising diamond-bearing regions in Russia is the Irkutsk region, where the search for gems was stopped in 1980 due to insufficient funding and negative results obtained in the southern part of the region.
In 2015, a number of scientists conducted an analysis that suggests that the Orenburg region has prospects for the presence of diamond-bearing regions.