Bacteria, fungi, algae and protozoa have much in common, which is further evidence of evolution. Artificial synthesis of lichen Mushrooms represent a symbiosis of fungi and algae
In the previous chapter, we learned about the relationship between plants and microbes, which is beneficial to both parties and is called symbiosis. Let's take a closer look at some aspects of this union.
Leguminous plants can produce sugars through photosynthesis, but are unable to assimilate atmospheric nitrogen. Nodule bacteria, on the contrary, cope well with this task, but cannot synthesize sugars because they do not have chlorophyll. But when these two organisms unite and exchange the products they produce, their life is assured.
Alder roots also contain nodules in which microbes live that absorb nitrogen from the air. This is also an example of symbiosis, as in legumes.
Extremely interesting plants are lichens. In the polar tundra, this is almost the only food of herbivorous animals. They are interesting because they represent combinations of fungi and algae: among the fungal cells live smaller cells of green or blue-green algae.
The body of a lichen of one type or another usually contains one permanent type of algae. True, some lichens growing in the alpine zone have two types of algae belonging to completely different groups (one type is green, the other is blue-green algae), and here we already encounter a triple symbiosis: mushroom + green algae + blue-green algae. In this case, blue-green algae plays a special role, since it provides carbon nutrition to the rest of the system through photosynthesis and absorbs nitrogen from the atmosphere.
Lichenologists (lichenology - the science of lichens) managed to isolate both partners from lichens - both the fungus and the algae - and grow them separately in pure cultures. From such pure cultures, they carried out the reverse “synthesis” of these organisms into lichens, which is schematically depicted in the figure.
Using radioactive carbon 14 C, it was proven that algae provide carbohydrate food for lichens. The latter bind carbon dioxide during photosynthesis, produce sugars from carbon dioxide and water and transport them to fungal cells. In one of the experiments, it was found that already 45 minutes after the arrival of radioactive carbon in the fungal cells, 60% of the carbon that had passed through the process of photosynthesis was found.
Swedish researcher K. Mosbach from Lund University describes the rate of synthesis of relatively complex gyrophoric acid by lichens. Within a minute after the arrival of radioactive carbon dioxide, carbon 14 C was found in its composition. This can be explained by the fact that radioactive carbon was first absorbed by algae cells and then, during photosynthesis reactions, was included in the composition of sugar molecules. The sugar molecules were transferred to the fungal cells of the lichen and there, under the influence of enzymes, they were first decomposed into simpler compounds with diatomic carbon, and then, with the assistance of other enzymes, gyrophoric acid was formed from them, containing 24 carbon atoms in its molecule. The entire path of radioactive carbon atoms can be simplified as follows:

A biochemist would carry out the complex processes of photosynthesis, decomposition and re-synthesis through many stages and use at least 10 enzymes to carry out individual chemical reactions. But in the cells of microorganisms all these operations are performed in less than a minute; after a minute, the first products - gyrophoric acid molecules - are ready. How primitive and imperfect is the automated conveyor belt in our factories in comparison with the “production” of this substance in nature! At the same time, we must not forget that at the same time and in the same cells, hundreds of other chemical reactions are taking place in perfect harmony!
Algae in lichens are capable of carrying out the process of photosynthesis at an external temperature of -5° C, and in some cases even at a temperature of -24° C.
As the experiments of lichenologists have shown, the algae also supplies its fungal “partner” with vitamins, and the blue-green algae also supplies nitrogen food. The fungus, for its part, supplies the algae with aqueous solutions of mineral salts and provides protection from the adverse effects of the external environment.
Nevertheless, it seems that algae are a kind of captives and forced labor for fungi. When separating partners from each other, mushrooms require “artificial” nutrition, while green and blue-green algae are completely independent organisms and themselves synthesize all the necessary organic compounds.
There are many other examples in nature of the cohabitation of microbes with other organisms. Fungal hyphae live in the soil on the roots of trees and penetrate into the root tissue. Mushrooms are constant companions of these trees. It turns out that their life on the roots is of great importance for tree species. Plants release carbohydrates into the soil through their roots, which are used by fungi. Hyphae also penetrate into the roots, but the plant regulates their activity in the root system, and the apical cells of the hyphae are sometimes dissolved by substances contained in the secretions of the roots. Plants, in turn, use substances found in the hyphae, and thus fungi contribute to their nutrition to a certain extent. This cohabitation of fungi with plants is called mycorrhiza. This connection is well known to mushroom pickers who collect the fruiting bodies of mycorrhizal mushrooms - porcini, boletus, and chanterelles. Fruiting bodies grow from mycelium (plexuses of hyphae located in the soil in close contact with tree roots). Therefore, we most often find porcini mushrooms under oak trees, boletus mushrooms under birch trees, and boletus mushrooms under aspen trees.
The first success in this field belongs to E. Thomas, who in 1939 in Switzerland obtained from myco- and photobionts the lichen Cladonia capillary with clearly visible fruiting bodies. Unlike previous researchers, Thomas carried out the synthesis under sterile conditions, which inspires confidence in the result he obtained. Unfortunately, his attempts to repeat the synthesis in 800 other experiments failed.
V. Akhmadzhyan’s favorite object of research, which brought him worldwide fame in the field of lichen synthesis, is Cladonia comb. This lichen is widespread in North America and has received the popular name “British soldiers”: its bright red fruiting bodies really resemble the scarlet uniforms of English soldiers during the war of the North American colonies for independence.
Small lumps of the isolated mycobiont Cladonia crestata were mixed with a photobiont isolated from the same lichen. The mixture was placed on narrow mica plates, soaked in a mineral nutrient solution and fixed in closed flasks. Strictly controlled conditions of humidity, temperature and light were maintained inside the flasks. An important condition of the experiment was the minimum amount of nutrients in the medium.
How did the lichen partners behave in close proximity to each other? The algae cells produced a special substance that “glued” the fungal hyphae to them, and the hyphae immediately began to actively entwine the green cells. Groups of algal cells were held together by branching hyphae into primary scales. The next stage was the further development of thickened hyphae on top of the scales and their release of extracellular material, and as a result, the formation of the upper crustal layer. Even later, the algal layer and the core differentiated, just like in the thallus of a natural lichen. These experiments were repeated many times in Akhmadzhyan’s laboratory and each time led to the appearance of a primary lichen thallus.

It would seem that one of the main mysteries of lichen has been solved: how it is formed from its constituent parts. But from further experiments it turned out that everything is not so simple.
A fungus isolated from Cladonia combata was placed next to algae from other lichens. Among them were green and blue-green photobionts isolated from 13 species of lichens, as well as free-living algae not found in lichen symbiosis. It turned out that mushroom hyphae take the “first steps of acquaintance” in the same way, that is, they entwine all algae and even... simple glass balls with the diameter 10-15 microns! But the next stages of “lichenization” of algae occurred differently, depending on the algal partner.
Later, in the same laboratory, they synthesized another lichen, Usnea bristles, and noted the same trends. The hyphae of the mycobiont with equal success began to entwine not only the cells of their own (Symbirthic) algae, but also Trebuxia remarkable, characteristic, as we already know, of other species of lichens. But if our own, native algae looked healthy and green between the mushroom threads, and after five months the thallus itself resembled sleepy, then the alien algae surrounded by the mycobiont were pale, yellow-green, and the thallus did not have the filamentous structure characteristic of this lichen.
Experiments on the synthesis of lichens are also being carried out. at Tel Aviv University under the guidance of Professor M. Galun. This researcher synthesized xanthoria wallae by placing myco- and photobionts of this plant side by side on agar. The symbionts lived side by side for eight to twelve months. During this time, pale yellow-orange lichen lobes with a diameter of 2-5 mm developed. Apothecia with spores also formed. True, in terms of their anatomical structure, the blades of artificial lichen were not identical to the blades of natural ones.
Attempts to divide a lichen into a fungus and an alga have been made for a long time, but most often ended in failure: even if sterility conditions were observed, it was not always certain that the resulting culture was a lichen symbiont and not an internal parasite of the lichen. In addition, experiments usually could not be repeated, but reproducibility is one of the main requirements for an experiment. But in the middle of the 20th century, a standard method was developed and several dozen lichen fungi (mycobionts) and lichen algae (photobionts) were isolated. Much credit for this work belongs to the American scientist V. Akhmadzhyan.
So, isolated lichen symbionts settled in laboratories, in sterile test tubes and flasks with a nutrient medium. Having pure cultures of lichen partners at their disposal, scientists decided on the most daring step - the synthesis of lichen in the laboratory. The first success in this field belongs to E. Thomas, who in 1939 in Switzerland obtained from myco- and photobionts the lichen Cladonia capillary with clearly visible fruiting bodies. Unlike previous researchers, Thomas performed the synthesis under sterile conditions, which inspires confidence in his result. Unfortunately, his attempts to repeat the synthesis in 800 other experiments failed.
V. Akhmadzhyan’s favorite object of research, which brought him worldwide fame in the field of lichen synthesis, is Cladonia comb. This lichen is widespread in North America and has received the common name "British soldiers": its bright red fruiting bodies are reminiscent of the scarlet uniforms of English soldiers during the war of the North American colonies for independence. Small lumps of the isolated mycobiont Cladonia crestata were mixed with a photobiont extracted from the same lichen. The mixture was placed on narrow mica plates, soaked in a mineral nutrient solution and fixed in closed flasks. Strictly controlled conditions of humidity, temperature and light were maintained inside the flasks. An important condition of the experiment was the minimum amount of nutrients in the medium. How did the lichen partners behave in close proximity to each other? The algae cells secreted a special substance that “glued” the fungal hyphae to them, and the hyphae immediately began to actively entwine the green cells. Groups of algal cells were held together by branching hyphae into primary scales. The next stage was the further development of thickened hyphae on top of the scales and their release of extracellular material, and as a result, the formation of the upper crustal layer. Even later, the algal layer and the core differentiated, just like in the thallus of a natural lichen. These experiments were repeated many times in Akhmadzhyan’s laboratory and each time led to the appearance of a primary lichen thallus.
In the 40s of the 20th century, the German scientist F. Tobler discovered that for the germination of Xanthoria wallae spores, the addition of stimulating substances is required: extracts from tree bark, algae, plum fruits, some vitamins or other compounds. It was suggested that in nature the germination of some fungi is stimulated by substances coming from algae.
It is noteworthy that for a symbiotic relationship to occur, both partners must receive moderate or even meager nutrition, limited humidity and lighting. Optimal conditions for the existence of a fungus and algae do not stimulate their reunification. Moreover, there are cases where abundant nutrition (for example, with artificial fertilizer) led to the rapid growth of algae in the thallus, disruption of the connection between symbionts and death of the lichen.
If we examine sections of the lichen thallus under a microscope, we can see that most often the alga is simply adjacent to fungal hyphae. Sometimes the hyphae are closely pressed against the algal cells. Finally, fungal hyphae or their branches can penetrate more or less deeply into the algae. These projections are called haustoria.
Coexistence also leaves an imprint on the structure of both lichen symbionts. Thus, if free-living blue-green algae of the genera Nostoc, Scytonema and others form long, sometimes branching filaments, then in the same algae in symbiosis the filaments are either twisted into dense balls or shortened to single cells. In addition, differences in the size and arrangement of cellular structures are noted in free-living and lichenized blue-green algae. Green algae also change in a symbiotic state. This primarily concerns their reproduction. Many of the green algae, living “in freedom”, reproduce by mobile thin-walled cells - zoospores. Zoospores are usually not formed in the thallus. Instead, aplanospores appear - relatively small cells with thick walls, well adapted to dry conditions. Of the cellular structures of green photobionts, the membrane undergoes the greatest changes. It is thinner than that of the same algae “in the wild” and has a number of biochemical differences. Very often, fat-like grains are observed inside the symbiotic cells, which disappear after the algae are removed from the thallus. Speaking about the reasons for these differences, we can assume that they are associated with some kind of chemical effect of the algae’s fungal neighbor. The mycobiont itself is also influenced by its algal partner. Dense lumps of isolated mycobionts, consisting of closely intertwined hyphae, do not look at all like lichenized fungi. The internal structure of the hyphae is also different. The cell walls of hyphae in a symbiotic state are much thinner.
So, life in symbiosis encourages the algae and the fungus to change their external appearance and internal structure.
What do cohabitants get from each other, what benefits do they derive from living together? The algae supplies the fungus, its neighbor in the lichen symbiosis, with carbohydrates obtained during the process of photosynthesis. An algae, having synthesized one or another carbohydrate, quickly and almost entirely gives it to its mushroom “companion”. The fungus receives not only carbohydrates from the algae. If the blue-green photobiont fixes atmospheric nitrogen, there is a rapid and steady outflow of the resulting ammonium to the fungal neighbor of the algae. The algae, obviously, simply gets the opportunity to spread widely throughout the Earth. According to D. Smith, “the most common algae in lichens, Trebuxia, very rarely lives outside the lichen. Inside the lichen, it is perhaps more widespread than any genus of free-living algae. The price for occupying this niche is supplying the host fungus with carbohydrates.”
When using site materials, it is necessary to place active links to this site, visible to users and search robots.
It is believed that mutualism (mutually beneficial symbiosis) of two types of living beings should form gradually, as a result of long co-evolution. However, experiments by American biologists have shown that many species of fungi and unicellular algae can form mutualistic systems almost instantly, without a previous period of mutual adaptation and without any genetic modifications. To do this, the fungus and algae must find themselves in an environment where they will be each other’s only sources of necessary substances, such as carbon dioxide and ammonium. The study confirmed the “ecological correspondence” hypothesis, according to which not all mutualistic systems existing in nature should be interpreted as the result of long-term previous coevolution.
Obligate (obligatory) mutualism is a mutually beneficial relationship between two species that cannot exist without each other. It is generally accepted that such relationships are formed gradually, during long-term coevolution and mutual adaptation, the “grinding in” of organisms to each other. Undoubtedly, in many cases this was the case (see N. Provorov, E. Dolgikh, 2006. Metabolic integration of organisms in systems of symbiosis).
Of course, not every species is able to integrate into a new environment. During introduction, a kind of sorting occurs, during which some newcomers take root in a new place, while others die. One way or another, we have to admit that an integral and interconnected community can be formed not only due to the co-evolutionary “grinding in” of species with each other over millions of years, but also due to the selection from among random migrants of species that successfully complement each other and get along well together. This idea, known as ecological fitting, has been developed by the famous American ecologist Daniel Janzen since the 1980s.
Can obligate-mutualistic systems, usually considered something like the apotheosis of coevolution, be formed according to the same scheme, that is, without any coevolution - simply due to the random correspondence of two accidentally encountered species, which, under certain conditions, turn out to be unable to live without each other? Experiments conducted by biologists from Harvard University (USA) allow us to answer this question in the affirmative.
The authors worked with the common baker's budding yeast Saccharomyces cerevisiae and the equally common unicellular algae Chlamydomonas reinhardtii. In nature, these species have not been observed in mutualistic relationships. In the laboratory, however, they formed an inextricable bond easily and quickly, without any evolution or genetic modification. To do this, it turned out to be enough to grow yeast and chlamydomonas without access to air in an environment where glucose is the only source of carbon and potassium nitrite is the only source of nitrogen.
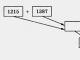
The scheme of mutualistic relationships between yeast and Chlamydomonas is quite simple (Fig. 1). Yeast feeds on glucose and produces carbon dioxide, which is necessary for chlamydomonas for photosynthesis (chlamydomonas do not know how to use the glucose contained in the medium). Algae, for their part, reduce nitrite, converting nitrogen into a form accessible to yeast (ammonium). Thus, yeast provides carbon to Chlamydomonas, and Chlamydomonas provides nitrogen to yeast. Under such conditions, neither species can grow without the other. This is obligate mutualism.
The authors were convinced that the mutualistic system grows safely in a wide range of glucose and nitrite concentrations, although neither of the two species survives alone under these conditions. Only with a very strong decrease in the concentration of glucose or nitrite does the growth of the mixed culture stop.
If you uncork the system, that is, give it access to atmospheric CO2, you get a community in which only one of the participants (yeast) cannot live without the other, while the second participant (Chlamydomonas) no longer needs the first to survive. However, even in this case, Chlamydomonas grows better in the presence of yeast than without it (obviously, the additional CO2 released by the yeast benefits them). Thus, the system remains mutualistic, although on the algae side the mutualism is no longer obligate. Neither species displaces the other.
If you add ammonium to the medium, the situation is reversed: now the yeast can live without algae (and does not need it at all), while the algae still cannot live without yeast. This is no longer mutualism, but commensalism (freeloading on the part of algae). In this case, yeast, which reproduces faster than algae, fills the entire living space, driving Chlamydomonas to extinction. The authors suggest that the stability of such asymmetric systems (in which only one of the participants is highly dependent on the other) is determined by the ratio of reproduction rates. If a dependent species reproduces faster than an independent one, then the cohabitation of the two species can be stable; otherwise, the independent species may completely displace its partner.
The authors conducted similar experiments with other species of Chlamydomonas and ascomycete fungi. It turned out that almost all types of yeast under these conditions form obligate-mutualistic relationships with Chlamydomonas. True, the productivity (growth rate) of symbiotic complexes turns out to be different. It was not possible to determine what it depends on: the authors did not find a connection either with the tendency of yeast to oxygen respiration or oxygen-free metabolism (fermentation), or with the natural habitats of the yeast, or with the rate of reproduction, or with the degree of influence of nitrite concentration on yeast growth. Obviously, the matter is in some other characteristics of the studied species.
The unicellular alga Chlorella refused to enter into a mutualistic relationship with yeast, because it itself can feed on glucose and in a mixed culture displaces yeast. The yeast Hansenula polymorpha did not form obligate-mutualistic complexes with algae, because they themselves are able to use nitrite as a source of nitrogen. But still, the study showed that a variety of species of ascomycetes and chlamydomonas are ready to enter into a symbiotic relationship with each other, once in suitable conditions.
Of the multicellular (more precisely, filamentous hyphae-forming) ascomycetes, two classic laboratory objects were tested - Neurospora crassa and Aspergillus nidulans. Both species are able to reduce nitrite and therefore do not form obligate-mutualistic systems with Chlamydomonas. However, genetically modified strains of these fungi, deprived of the ability to utilize nitrite, entered into symbiosis with algae in the same way as yeast. As it turned out, in this case, chlamydomonas cells come into direct physical contact with fungal hyphae: under a microscope, hyphae are visible, covered with chlamydomonas, like a Christmas tree (Fig. 2).
Mutualistic relationships between Chlamydomonas and yeast also apparently require the establishment of physical contacts between cells. This is evidenced by the fact that systematic shaking of a mixed culture of yeast and algae sharply slows down the growth of the symbiotic system.
Using an electron microscope, the authors discovered tight junctions formed between the cell walls of Aspergillus nidulans and Chlamydomonas reinhardtii, and the algal cell wall at the points of contact becomes thinner - possibly under the influence of enzymes secreted by the fungus.
Similar intercellular contacts are characteristic of classical fungal-algal symbiotic systems - lichens. During their evolution, ascomycetes many times entered into symbiosis with algae and cyanobacteria, forming lichens. Lichen-forming groups are scattered throughout the phylogenetic tree of ascomycetes. This means that such evolutionary events occurred repeatedly and independently in different evolutionary lineages of fungi (see F. Lutzoni et al., 2001. Major fungal lineages are derived from lichen symbiotic ancestors). Apparently, ascomycetes in general are “predisposed” (preadapted) to the formation of mutualistic complexes with unicellular algae. Experiments by American scientists may shed light on the early stages of the formation of such complexes.
However, one should not overestimate the similarity of the experimentally obtained mutualistic systems with lichens. If only because in most lichens, only the fungal component cannot live alone, while photosynthetic components (unicellular algae and cyanobacteria), as a rule, can live perfectly well without a fungus. That is, lichens are not obligate-mutualistic systems. And lack of access to atmospheric CO2 is hardly a problem that algae often have to face in nature. The main thing in the work under discussion is the demonstration of the general principle. The study showed that obligate mutualism can develop instantly, without any evolution - simply due to the fact that changing conditions make species interdependent. Of course, in order for something truly complex and highly integrated, like a lichen, to develop from such a hastily formed symbiotic complex, millions of years of coevolution are no longer necessary.